Phase diagrams depict phase relationships within multi-component systems. Phases are physically separate regions of homogeneous chemistry. Possible phases include a liquid phase (multiple liquid phases if the liquids are immiscible), a gas phase, and multiple solid phases. Solid phases may exhibit solid solution, i.e., have a range of compositional variation.
To introduce phase diagrams, we will consider the characteristics of a series of phase diagrams of increasing complexity, beginning with a binary mixture of diopside and anorthite, at 1 atm pressure.
Figure 6-1
Some important elements of this phase diagram include its:
Eq 6-1:![]()
![]()
Eq 6-2:![]()
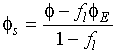
In these and the following equations f![]() is the fraction of liquid and
is the fraction of liquid and ![]() is the composition with the
subscripts E, s and l denoting the eutectic liquid, the solid and the
liquid,
respectively. Melting will continue until the composition of the residual solid reaches the value
1, i.e., the diopside component has melted completely and the residual solid is pure anorthite. Once this happens the system is no longer restricted to
the eutectic point and the heat applied to system will again increase
temperature. For this example, the critical fraction of liquid is
0.69.
is the composition with the
subscripts E, s and l denoting the eutectic liquid, the solid and the
liquid,
respectively. Melting will continue until the composition of the residual solid reaches the value
1, i.e., the diopside component has melted completely and the residual solid is pure anorthite. Once this happens the system is no longer restricted to
the eutectic point and the heat applied to system will again increase
temperature. For this example, the critical fraction of liquid is
0.69.
As temperature rises again, once this critical fraction is reached, the liquid composition follows
the liquidus until the liquid is of starting composition An![]()
![]() .
The position of the liquidus indicates the composition of
liquid at a particular temperature. Thus when one of the two solids is present in equilibrium with a liquid, mass conservation requires that for an arbitrary initial bulk composition
.
The position of the liquidus indicates the composition of
liquid at a particular temperature. Thus when one of the two solids is present in equilibrium with a liquid, mass conservation requires that for an arbitrary initial bulk composition ![]() :
:
Eq 6-3:![]()
![]()
Eq 6-4:![]()
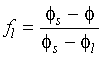
In geometric terms:
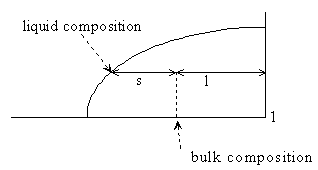
Figure 5-2. (the right hand lever should be labelled l, not 1)
and so this mass balance is the basis of the lever rule, i.e.
Eq 6-5:![]()
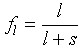
We call the situtation just described closed system, equilibrium melting. What if melt were continuously separated from residual solids instead? We call this open system melting. Until the critical fraction of liquid is reached, the system behaves in exactly the same way as before--the liquid composition is determined by the eutectic. However once diopside is gone only the phase anorthite remains which melts at 1553°C, a gap of 279°C. Obviously the energetics of melting for closed and open systems are quite different.
A number of solids exhibit solid solution, for example plagioclase, so that there is compositional variation in a single phase. The phase diagram for plagioclase is:
Figure 6-3
The solidus is no longer a horizontal line--the solid composition at equilibrium depends on the bulk composition of the system composition and the temperature. For a given bulk composition, one finds the solid and liquid proportions by applying a lever ruler:
Figure 6-4
As an example consider the cooling of material of composition An![]()
![]() .
The first crystals form at 1450°C, of composition ~An
.
The first crystals form at 1450°C, of composition ~An![]()
![]() . With
continued cooling the liquid composition follows the liquidus and the
solid composition follows the solidus until the original liquid is
fully crystallized at a temperature of about 1333°C. The last
liquid composition is about An
. With
continued cooling the liquid composition follows the liquidus and the
solid composition follows the solidus until the original liquid is
fully crystallized at a temperature of about 1333°C. The last
liquid composition is about An![]()
![]() .
.
It would be unusual at best for a natural solid to follow this closed system, equilibrium behavior. The solid initially formed will either be segregated by mechanical settling or protected from further reaction with the liquid by crystals formed later on the surface (a phenomenon called zoning). We call this path the path of fractional crystallization. In the following figure focus on the curves labelled TSC. These represent the integrated total solid composition obtained as a function of temperature when the solid produced at any instance is in equilibrium with the liquid composition at that temperature--more calcic solids produced early in the crystallization process do not back react with the liquid. The endpoints for the TSC are known, the initial value determined by the gap between the solidus and the liquidus and the final state by the bulk composition of the original liquid. The key differences from equilibrium crystallization are that the last liquid is much more sodium rich and liquid remains in a system to a much lower temperature.
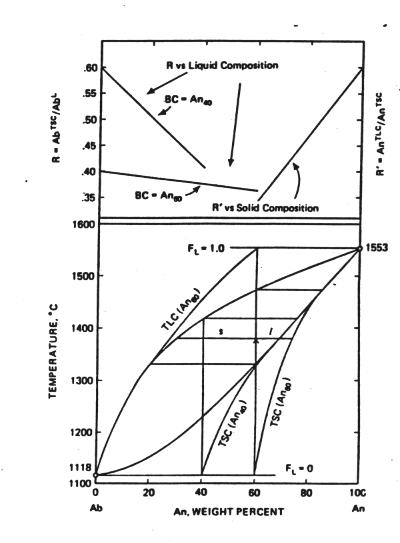
Figure 6-5
Ternary Systems
Multi-component phase relationships can be visualized on a triangular ternary plot. Consider an example involving the three components diopside, anorthite and albite (i.e., there are two phases: diopside and a solid solution of plagioclase).
Figure 6-6
There are several features of this diagram to note:
As an example of the application of this diagram, consider the cooling of material of a composition from within the diopside field, say of
composition Di![]()
![]() An
An![]()
![]() Ab
Ab![]()
![]() . As the initial liquid begins to cool, the liquidus is reached at
1300°C and diopside begins to crystallize. The composition of the residual liquid moves
straight down (away from the diopside vertex, because only diopside is
crystallizing). When the cotectic is
reached,
plagioclase also begins to crystallize with the liquid composition locked on
the cotectic. Of what composition is the first plagioclase formed? This
information can't be read from
the diagram; we need additional information. The answer is about An
. As the initial liquid begins to cool, the liquidus is reached at
1300°C and diopside begins to crystallize. The composition of the residual liquid moves
straight down (away from the diopside vertex, because only diopside is
crystallizing). When the cotectic is
reached,
plagioclase also begins to crystallize with the liquid composition locked on
the cotectic. Of what composition is the first plagioclase formed? This
information can't be read from
the diagram; we need additional information. The answer is about An![]()
![]() for this
composition, about 30% higher than liquid, consistent with what we might
have guessed from the phase diagram for plagioclase alone. Upon further cooling, the liquid composition follows the cotectic.
Travel down the cotectic stops when the solid has composition Ab=An, i.e., a liquid about
An
for this
composition, about 30% higher than liquid, consistent with what we might
have guessed from the phase diagram for plagioclase alone. Upon further cooling, the liquid composition follows the cotectic.
Travel down the cotectic stops when the solid has composition Ab=An, i.e., a liquid about
An![]()
![]() ,
about 30% lower than the final solid.
,
about 30% lower than the final solid.
Contrast the behavior of plagioclase alone and the situation just considered, that of diopside-saturated plagioclase
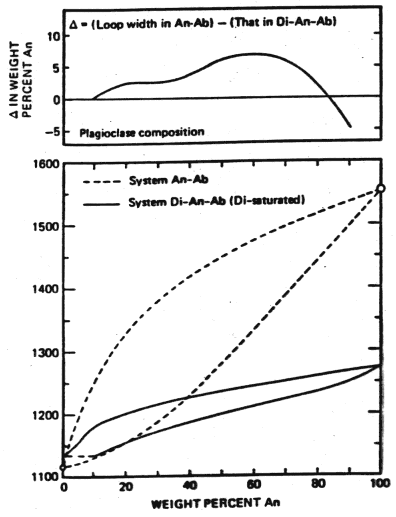
Figure 6-7
At 1200°C, for plagioclase alone the liquid is in
equilibrium with An![]()
![]() crystals contrasted with An
crystals contrasted with An![]()
![]() crystals for the diopside saturated system. There is a much lower temperature of melting across the entire compositional
range and as illustrated in the upper part of the figures, a smaller gap between liquid and solid compositions except at very high An content.
crystals for the diopside saturated system. There is a much lower temperature of melting across the entire compositional
range and as illustrated in the upper part of the figures, a smaller gap between liquid and solid compositions except at very high An content.
Returning to the question of crystallization paths in the system diopside-plagioclase, suppose that instead of starting in the diopside field we start in the plagioclase field. The liquid composition will move directly away from the solid plagioclase composition at that point in the crystallization path; see the diopside-plagioclase phase diagram. Thus, the liquid composition follows a curved path to the cotectic, with the tangent of the curve pointing down to the solid composition at that point in the crystallization sequence.
| Oceanography 540 Pages Pages Maintained by Russ McDuff (mcduff@ocean.washington.edu) Copyright (©) 1994-2002 Russell E. McDuff and G. Ross Heath; Copyright Notice Content Last Modified 10/14/2002 | Page Last Built 10/14/2002 |