The subsolidus phase relationships for this system are shown here:
![[Figure 7-1]](f12-4.gif)
Figure 7-1. From (18) (the Hess paper).
The tie-line between the
diopside vertex and the MgSiO![]() point on the Fo-Qtz boundary represents solid
solution of these components. The naturally occurring diopside is of the
composition marked a and the enstatite is of the composition marked
b. Note
the immiscibility gap (indicated by the dashed line) in this solid solution of pyroxenes. The diopside rich member is in the monoclinic crystal system while the enstatite rich member is in the orthorhombic crystal system, thus they are often referred to as clinopyroxene (cpx) and orthopyroxene (opx), respectively.
point on the Fo-Qtz boundary represents solid
solution of these components. The naturally occurring diopside is of the
composition marked a and the enstatite is of the composition marked
b. Note
the immiscibility gap (indicated by the dashed line) in this solid solution of pyroxenes. The diopside rich member is in the monoclinic crystal system while the enstatite rich member is in the orthorhombic crystal system, thus they are often referred to as clinopyroxene (cpx) and orthopyroxene (opx), respectively.
The liquidus at 2 GPa (20 kbar, ~67 km depth) is shown here:
![[Figure 7-2]](f12-5.gif)
Figure 7-2
The bulk composition of mantle peridotite is at the point labelled P--in terms of the minerals Fo, Di(a) and En(b) it has the composition 53:31:16.
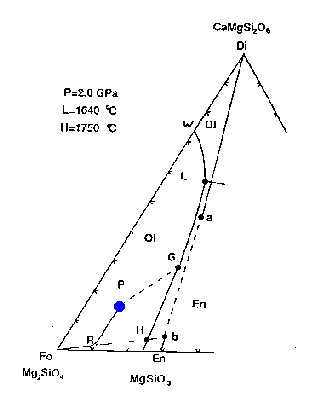
Figure 7-2a
How would melting proceed with addition of heat or adiabatic decompression? The first liquid composition will be at point L, providing that the three phases coexist. This is a peritectic point, i.e., it lies outside the Fo+Di(a)+En(b) triangle. The melting reaction at the peritectic is:
.07 Fo + .93 Di(a) ==> 0.8 liquid + 0.2 En(b)
The melt is generated mainly at the expense of diopside but also minor amounts of forsterite; additional enstatite is produced. The liquid composition stays at point L as long as the three phases are present, while the residual solid composition moves directly away from point L from point P toward point R.
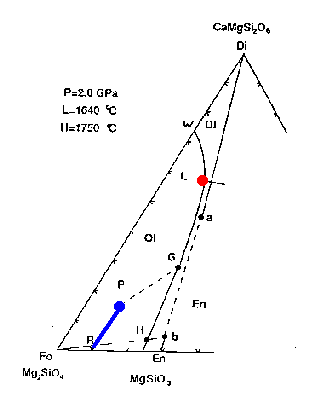
Figure 7-2b
Reaching R marks the point at which all Di(a) has been melted (the solid contains a combination of Fo and En(b).
The liquid composition then follows the Ol-En cotectic toward point G. Anywhere along this part of the liquidus, the intersection of the tangent to this cotectic and the Fo-En(b) tieline gives the proportions in the liquid generated, via the lever rule approximately 6 parts En(b) to 1 part Fo:
0.15 Fo + .85 En(b) ==> liquid
Thus the residual solid composition follows the Fo-En(b) tie from point R toward the Fo vertex. When the liquid composition reaches the point labeled G, the points Fo and P are in line and at this point no En(b) remains in the solid.
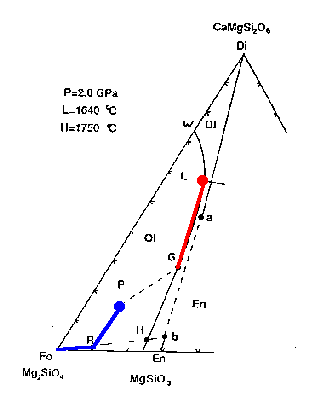
Figure 7-2c
The liquid composition begins to move toward the Fo vertex reaching its final composition P at 100% melting.
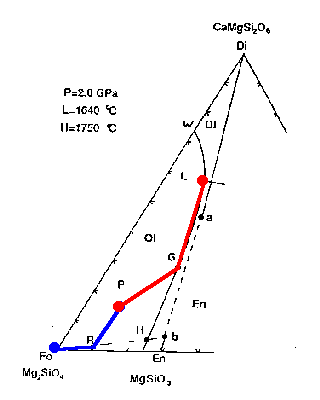
Figure 7-2d
To review we can watch the dance of the melting sequence.
In contrast to the equilibrium melting just discussed, fractional melting (remaining crystals are isolated from the liquid) generates a different relationship between melt produced and the temperature, as shown in Figure 7-3 below. Note the large increase in temperature (heat supply) required to undergo additional melting once diopside is all melted. In this way the amount of diopside in the original melt acts as a strong control on the degree of melting that the mantle undergoes.
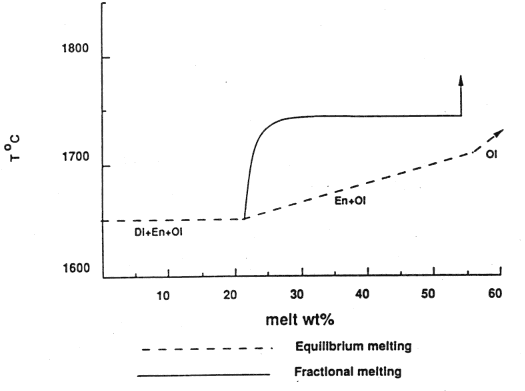
Figure 7-3.
The phase relationships we have been examining are pressure dependent, as this diagram of the liquidus at two different pressures shows:
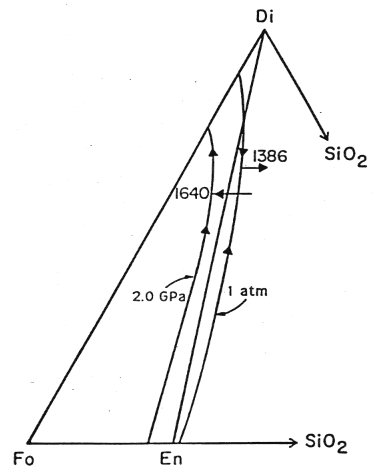
Figure 7-4.
In this diagram, the arrows on the liquidus show the direction of lower temperature. As pressure is reduced, the peritectic moves to a lower temperature (for the 20 kbar drop shown from 1640 to 1386 °C) and to a more Di and Qtz rich composition. If melt produced at the 2 GPa peritectic (point L in the earlier figure) is decompressed it will lie well into the Fo+liquid field. Thus when cooled, olivine will be the first phase to crysallize. The liquid composition will move away from the Fo vertex, intersecting the olivine-diopside cotectic at a point up from the peritectic. Diopside will then crystallize moving the liquid composition toward the 1 bar peritectic. When mantle is melted, the particular composition of the liquid generated will depend on the P-T regime and in particular the point at which the solidus is reached.
Al is not a component within the system Fo-Di-Qtz. Its introduction via melting of garnet is an additional secondary effect as the mantle melts. However its presence plays a much more important role in the crystallization history of the liquid as the basalts making up the crust are formed. At shallow levels in the lithosphere, it is more relevant to consider the system Fo-Di-An (plagioclase (represented by its endmember anorthite in this model is the principal aluminum-bearing phase in basalts):
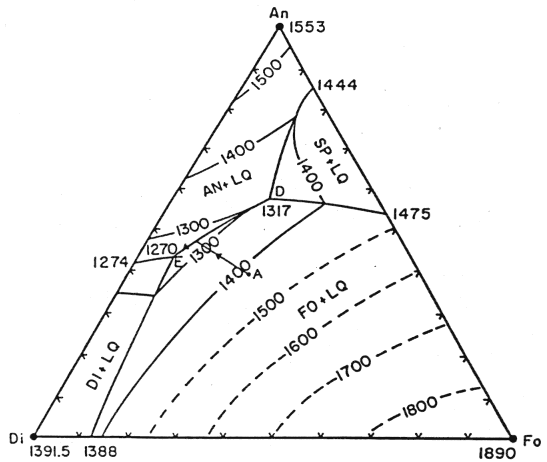
Figure 7-5.
A primitive melt formed in the mantle would have a composition near point A. That liquid would first crystallize olivine, then anorthite (Al bearing), then diopside. Petrographic studies of basalts reveal that crystallization of diopside never follows olivine directly, which restricts the possible composition of the parent melt, i.e., point A cannot be much lower in the diagram. While we will not develop the topic in more depth, it is through approaches of this kind that the melting history of the mantle is constrained. Important tools are the melting relationships affecting major elements and the partitioning of trace elements during melting events.
| Oceanography 540 Pages Pages Maintained by Russ McDuff (mcduff@ocean.washington.edu) Copyright (©) 1994-2002 Russell E. McDuff and G. Ross Heath; Copyright Notice Content Last Modified 10/14/2002 | Page Last Built 10/14/2002 |